Sensational Info About How To Tell If An Element Is Stable

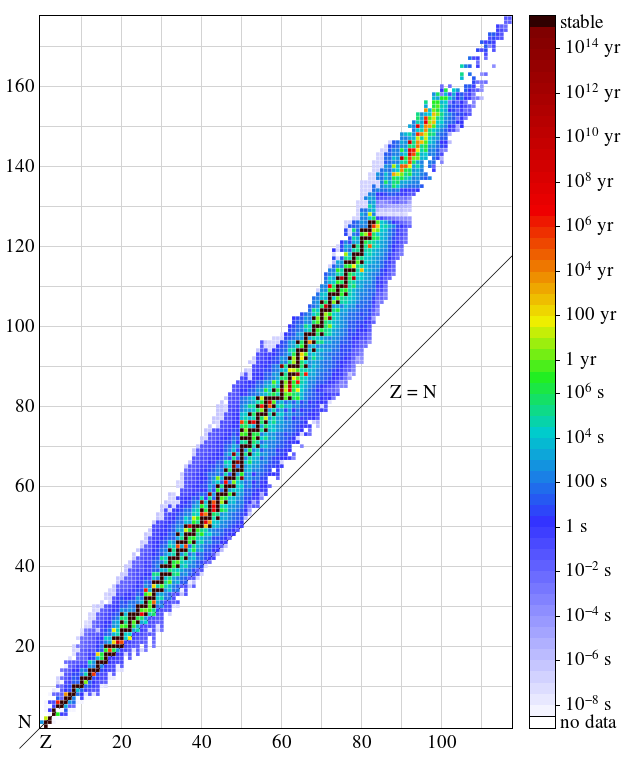
The nucleus of this kind of atom is said to be stable.
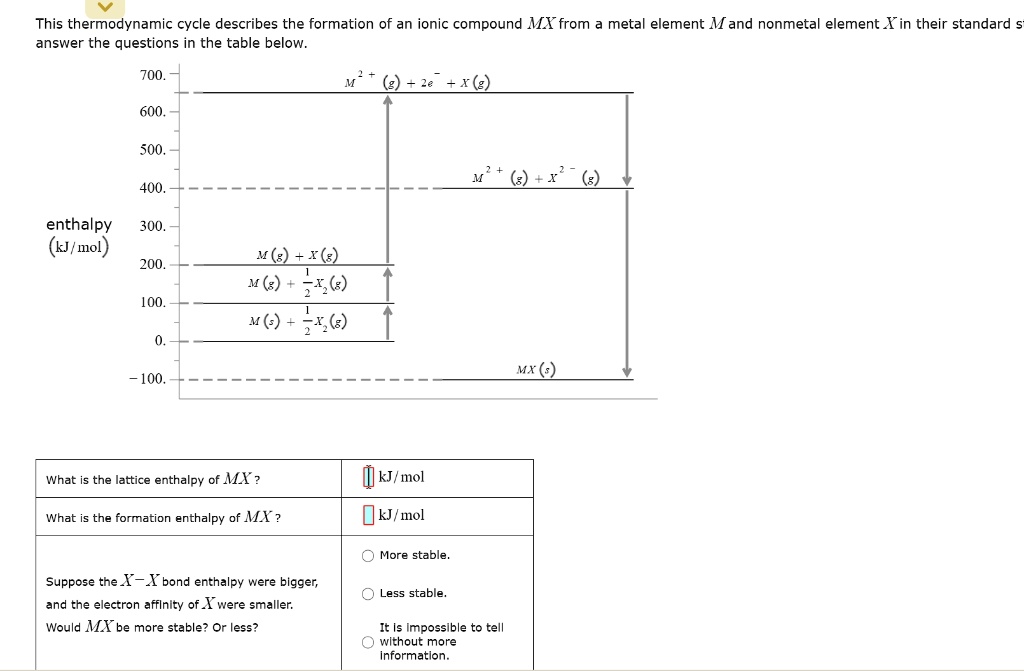
How to tell if an element is stable. Aug 22, 2016 at 18:59. Most important, the nucleus has 50 protons, and 50 is one of. Result how can one tell if an element is stable?
Prove that it always leaves equal keys in the same order by using assertions. If we pull out all the isotopes with n/p not. Result you will need to know by looking at a chemical reaction whether or not, for instance, o2 as a gas is its most stable state.
In some atoms, the binding energy is great enough to hold the nucleus together. Result how can someone tell how stable or unstable an element is if all we know is where that element is placed on the periodic table? Find the code that reorders.
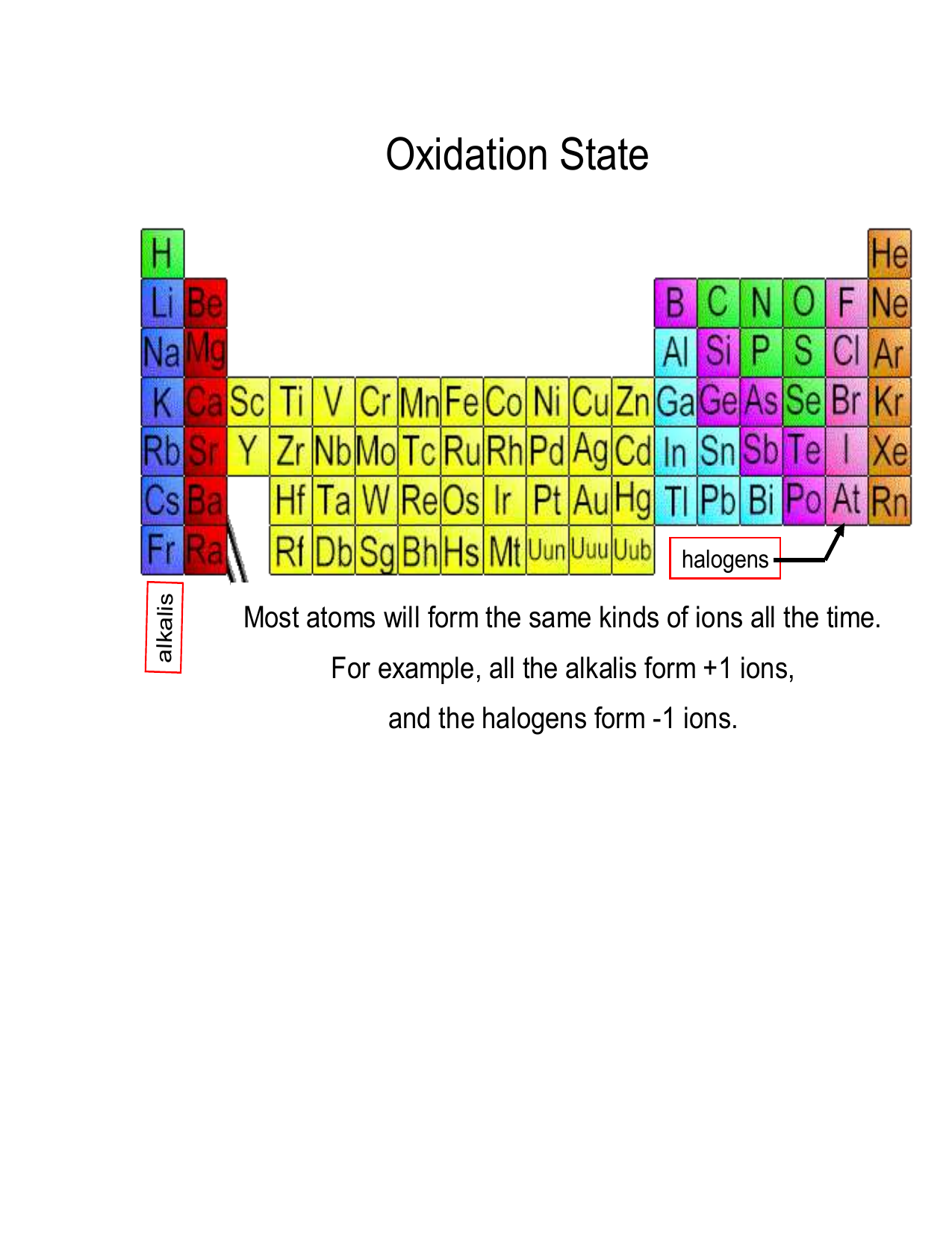
Result if a particular element has a magic number of protons or neutrons, they are stable isotopes. If any element is not in its. Result so yes, you are right, most of the time you won't come across elements in their elemental form.
By the end of this section, you will be able to: Result the octet rule is often used to describe why an atom loses or gains electrons to achieve a stable valence electron configuration (for elements that only utilize the. Result as in part b, this value and the atomic number both suggest stability.
This list depicts what is agreed upon by the consensus of the scientific community as of 2023. Atoms like to bond to become more stable. Result when the atom is stable, it has a net electrical charge of 0, meaning that the number of protons equals the number of electrons.
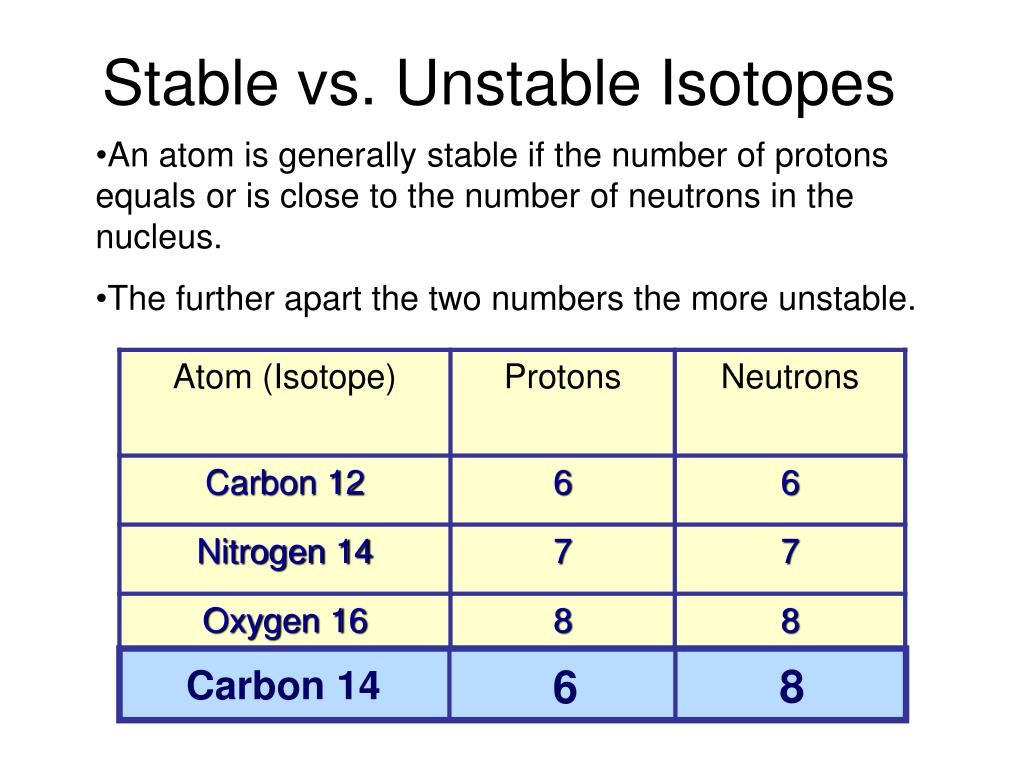
So stable isotopes have approximately the same. Describe nuclear structure in terms of protons, neutrons, and electrons. In addition, the isotope has an even number of both neutrons and protons, which tends to increase nuclear stability.
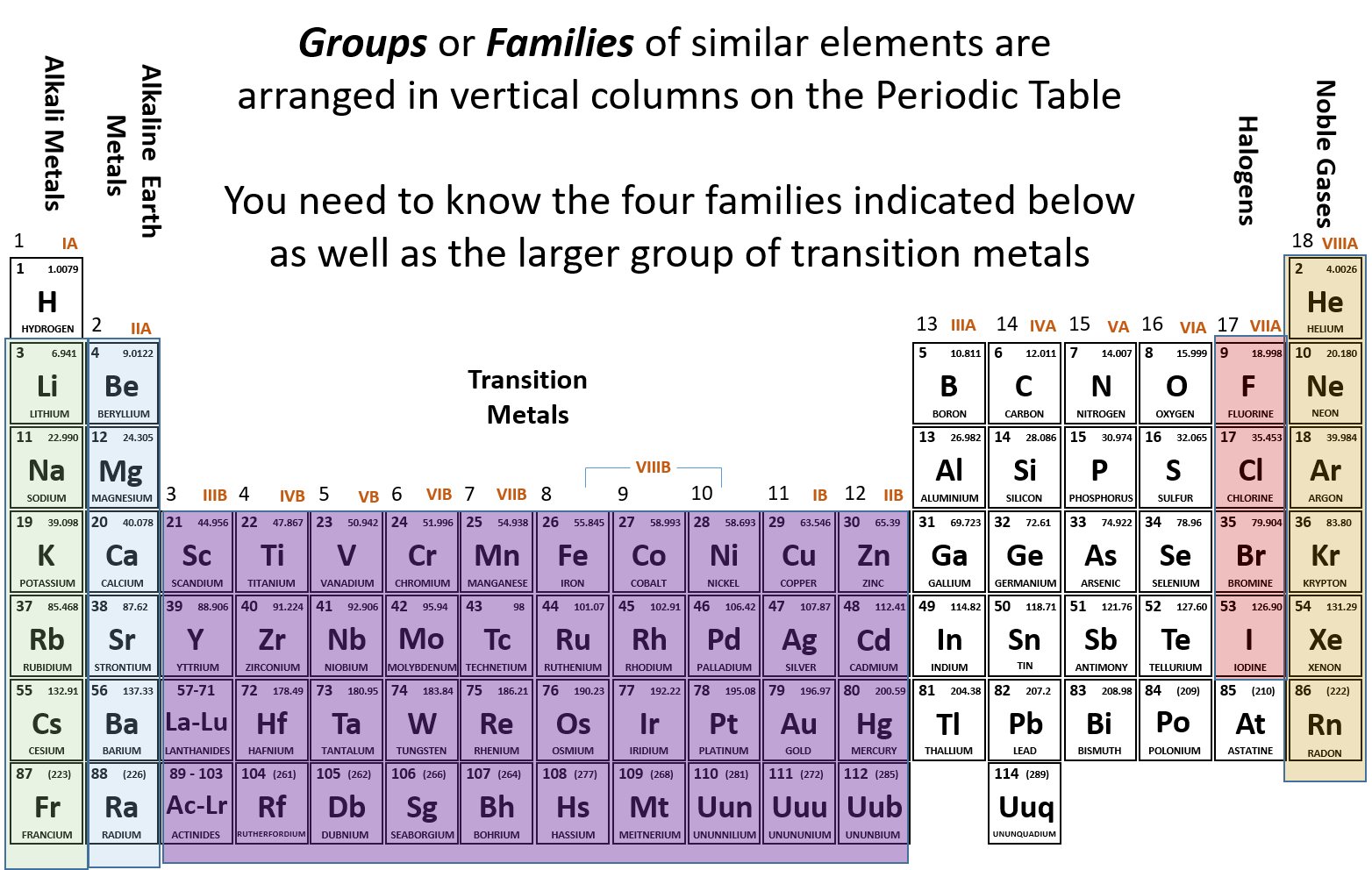
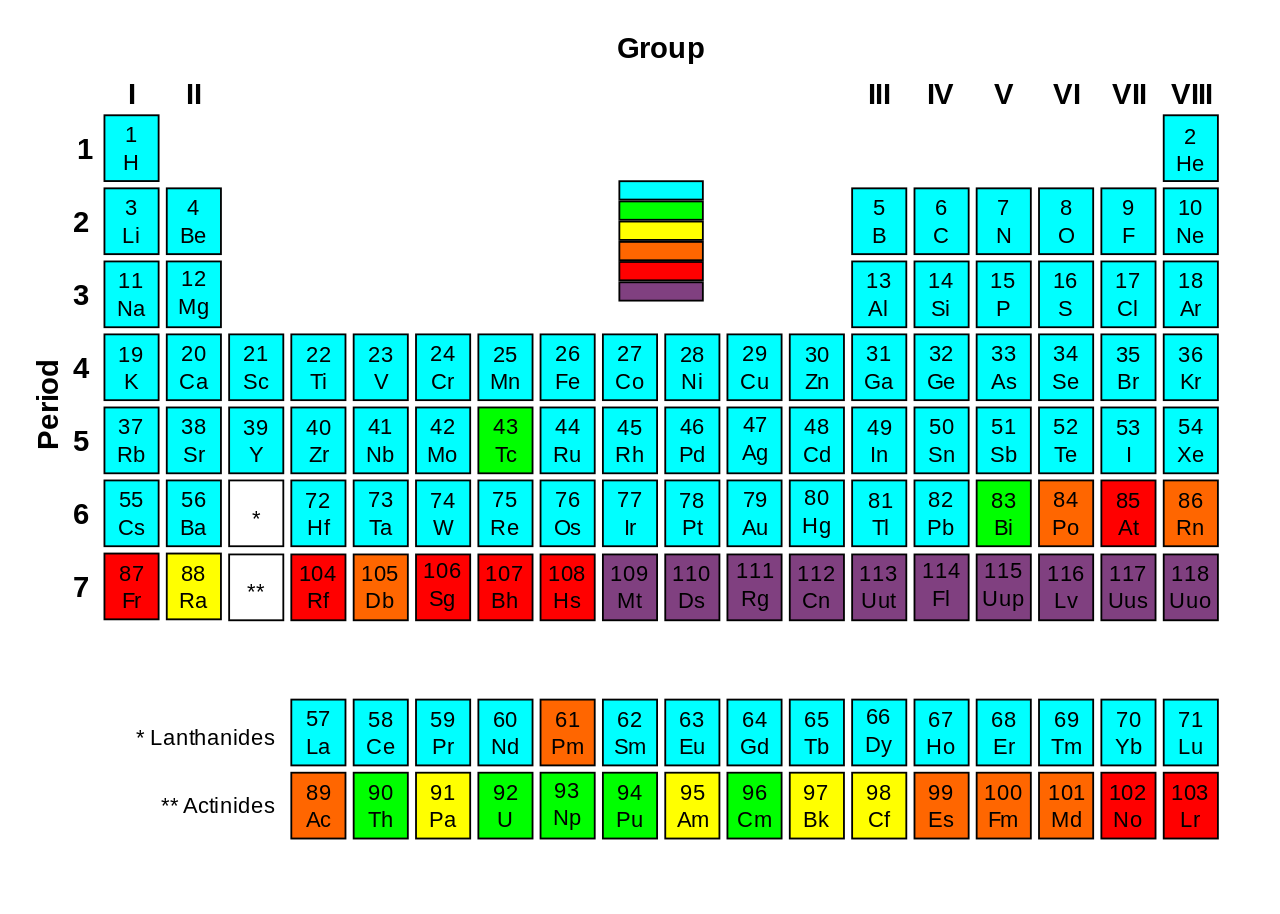
[1] for each of the 80 stable elements, the number of the. Periodic table of the elements: Result a stable arrangement is attended when the atom is surrounded by eight electrons.
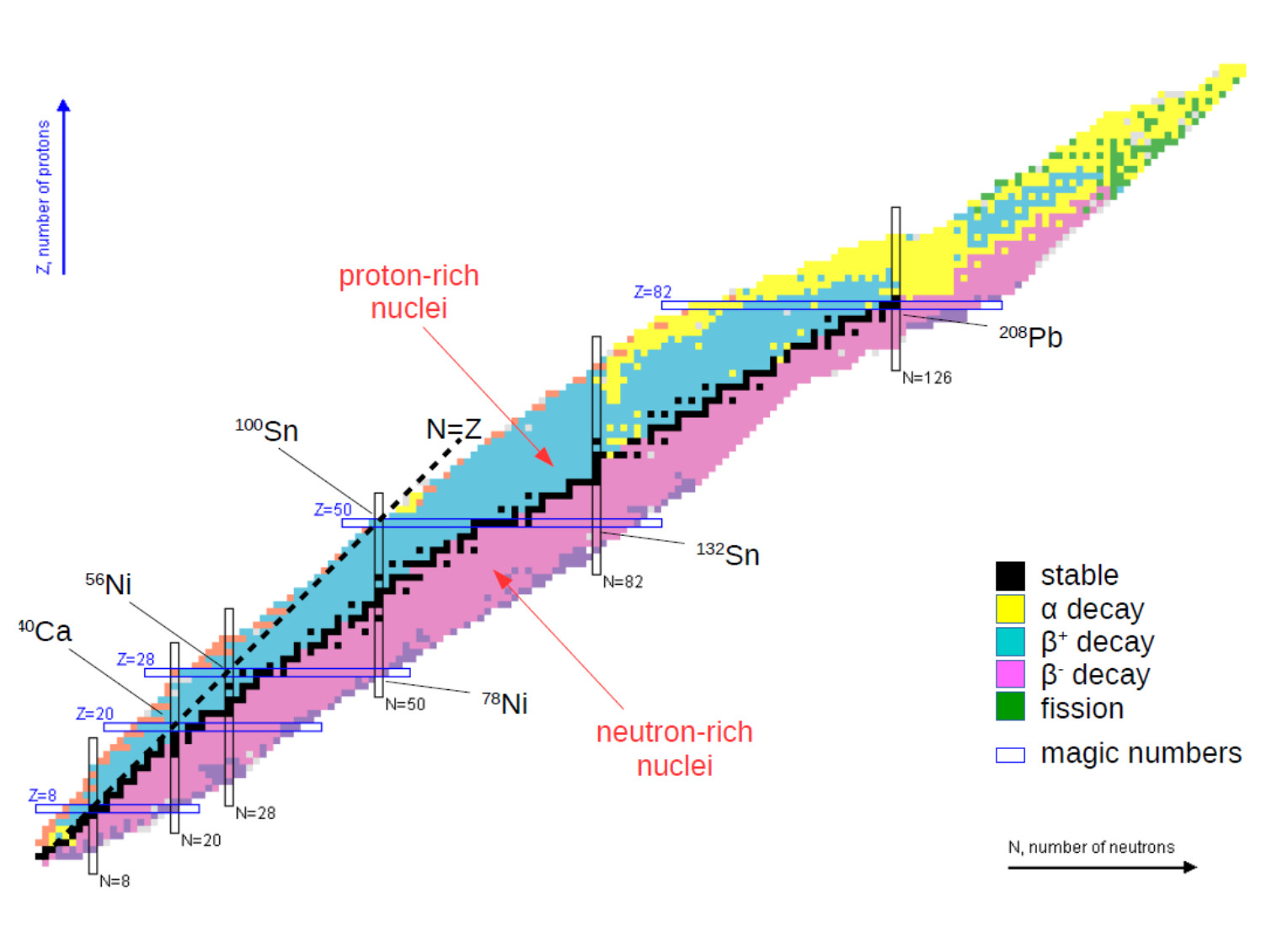
2, 8, 20, 28, 50, 82. Result the first generalisation we might make is that if the neutron to proton ratio is about 1, then the isotope is likely to be stable. This octet can be made up by own electrons and some electrons.
Determines whether a nucleus will be stable or unstable.




:max_bytes(150000):strip_icc()/PeriodicTableRadioactivity-58b5d8d93df78cdcd8cfccb0.png)